BonVie+
Innovative healing. Proven Results.
BonVie+ is a single-use osteoconductive implant intended to fill bony voids or gaps in the skeletal system, including the extremities and pelvis. Supplied as a kit, BonVie+ includes calcium salts, biocompatible and biodegradable polymers, and mixing solution, with a silicone mat for granulation into various sizes. Once implanted, the granules gradually resorb over 3–12 months, allowing bone to grow into the space and fully replace the implant during the healing process.
- Engineered for programmed resorption and optimal bone growth
- Matches natural bone mineral composition
- Designed to eliminate seromas and wound drainage
- Familiar, easy-to-use kit integrates seamlessly into workflow
- Improved bone healing may result in improved implant stability, contributing to longevity of implants due to lowered risk of loosening
- Highly osteoconductive1
- Robust compressive strength and elastic modulus2

Predictable Bone Restoration
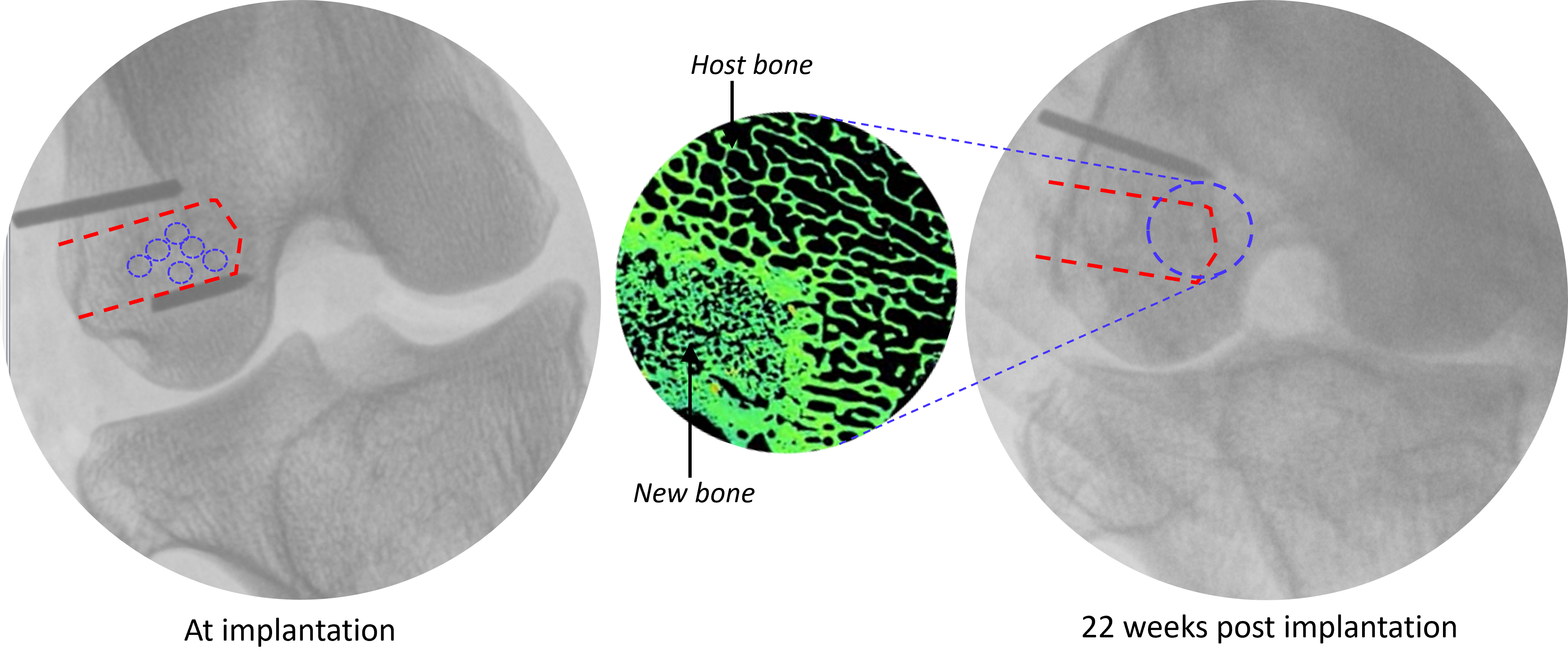
Synthetic bone graft substitutes most commonly are made from calcium sulfate, which resorbs fast without providing a competent scaffold, thus resulting in seromas and wound drainage. In contrast, BonVie+ is made using a combination of calcium carbonate, hydroxyapatite, and polymers which are engineered for programmed resorption without causing seromas and wound drainage.
EP Granules with Tobramycin, a similar product to BonVie+, delivers antibiotics at the infection site
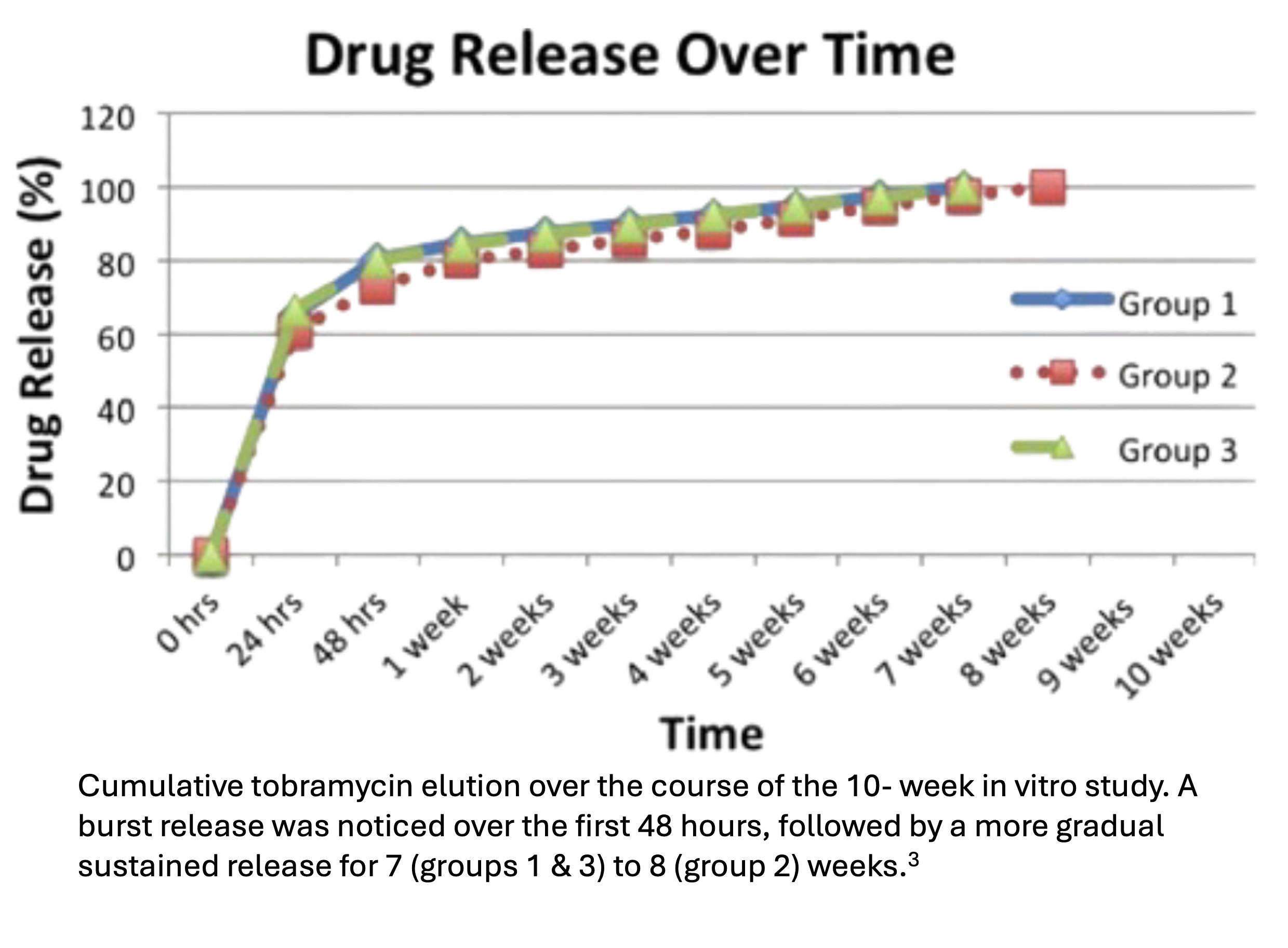
BonVie+ is based on EP Granules with Tobramycin, an investigative product identical to BonVie+ but pre-loaded with tobramycin. Antibiotic-loaded bone cement is frequently utilized to deliver antibiotics to the site of infection; however, bone cement is a nondegrading foreign body and known to leach its antibiotic load, after an initial burst release, at subtherapeutic concentrations for months. EP Granules with Tobramycin demonstrated extended bactericidal activity and resorbability in vitro.3 Preliminary results from IDE study of EP Granules with Tobramycin validate the game changing potential for simultaneously addressing infection and restoring bone growth in patients at 1-year follow up.4
REFERENCES
1. Antibiotic-Eluting Resorbable Bone-Void Filler Evaluated In A Large Animal Infection Prevention Model, Z. Ferrell, D.W. Grainger and K.D. Sinclair, European Cells and Materials Vol. 37 2019 (pages 265-276) DOI: 10.22203/eCM.v037a16.
2. Brooks BD, Sinclair KD, Davidoff SN, Lawson S, Williams AG, Coats B, Grainger DW, Brooks AE., 2013. Molded polymer-coated composite bone void filler improves tobramycin -releasing polycaprolactone–release kinetics. J Biomed Mater Res Part B 2013: 00B: 000–000.
3. Jones Z, Brooks AE, Ferrell Z, Grainger DW, Sinclair KD. 2015. A resorbable antibiotic eluting bone void filler for periprosthetic joint infection prevention. J Biomed Mater Res Part B 2015:00B:000–000.
4. https://clinicaltrials.gov/study/NCT05361941?term=elute%20&rank=4
All necessary regulatory documentation, instructions for use, published studies, patient education tools, etc. (as applicable) can be provided upon request by contacting cs@spartanmedical.com or (888) 240-8091. We are available at any time to meet with providers and staff to answer any questions about Spartan Medical’s entire portfolio of advanced medical solutions.
Looking for more Advanced Biologics and Dermis solutions?
Click on the below links to see our entire portfolio