AlloFuse® Select CM
Cellular Bone Matrix
AlloFuse® Select CM is a cellular allograft in a moldable bone matrix that is indicated for general bone grafting applications in orthopedic and spinal fusion procedures, offering similar clinical outcomes as autograft without the complications.1 On average, AlloFuse® Select CM delivers three million viable bone-forming cells per cc2 to the fusion site with greater than two-thirds of the cells being mesenchymal stem cells.3 Offering an alternative to autograft, AlloFuse® Select CM engages the body’s natural healing power to enhance bone growth and fusion potential with a moldable, compactable, granulated matrix.
- Tested to ensure consistent cellular viability
- Verified to have the osteogenic potential necessary for bone growth
- Contains a demineralized bone matrix with validated osteoinductive potential to initiate new bone growth4
- Contains 85% cell viability on average2
- Cellular component that is comprised, on average, of 68% mesenchymal stem cells (MSCs)3

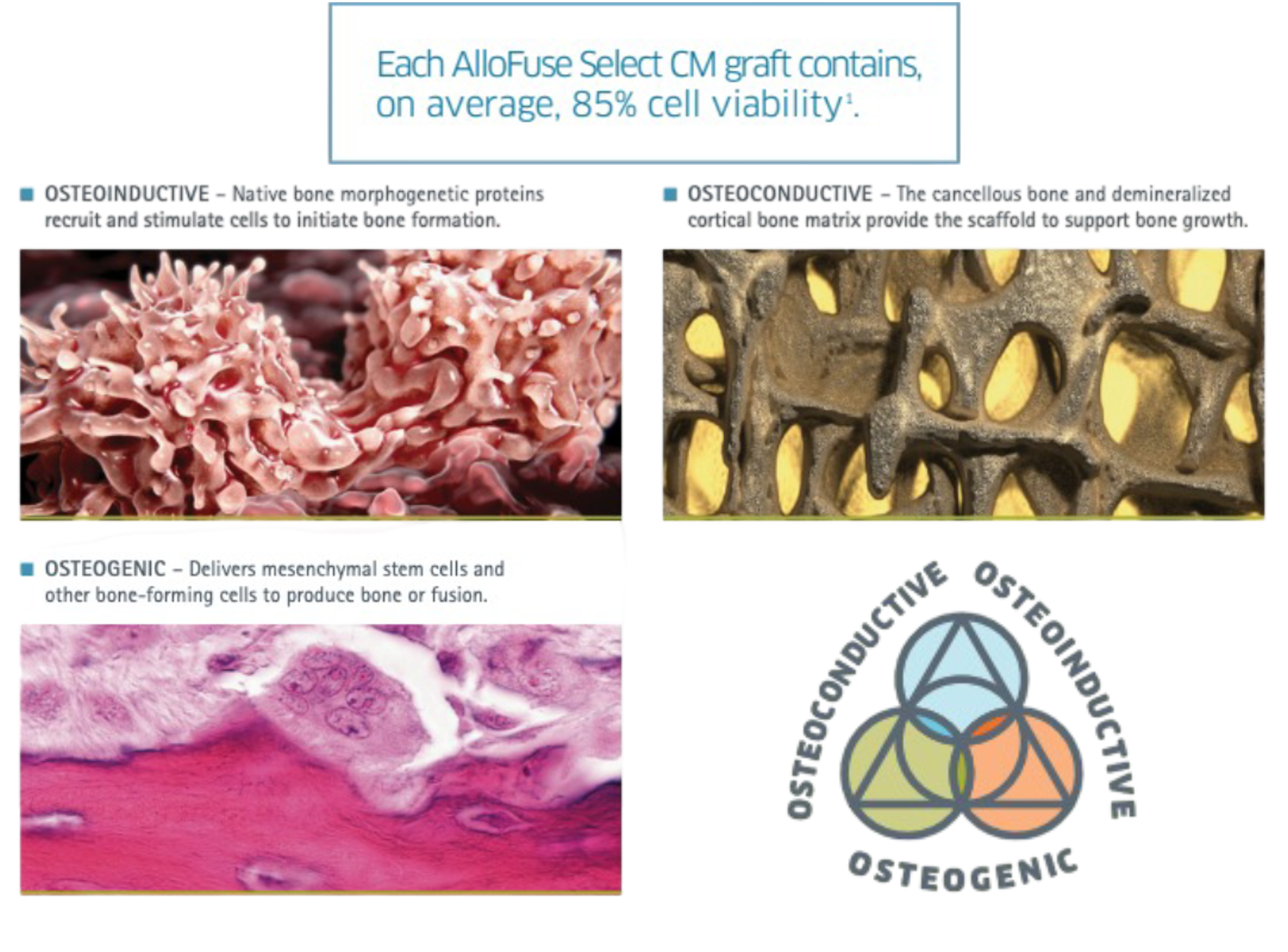
AlloFuse® Select CM combines natural osteoconductive, osteoinductive, and osteogenic properties with native, viable bone forming cells to help initiate and nurture bone growth delivering the benefits of autograft without the drawbacks. AlloFuse® Select CM delivers all three properties necessary to support the initiation and nurturing of bone growth at the fusion site of care.
- Osteoconductive: Cancellous bone and demineralized cortical bone matrix provides the scaffold to support bone growth
- Osteoinductive: Native bone morphogenetic proteins recruit and stimulate cells to initiate bone formation
- Osteogenic: Delivers mesenchymal stem cells and other bone-forming cells to produce bone
AlloFuse® Select CM engages the body’s natural healing power to enhance bone growth and fusion with a moldable, compactable, granulated matrix. Proven in over 25 peer-reviewed clinical papers and 250,000 surgical cases with no attributed adverse events5
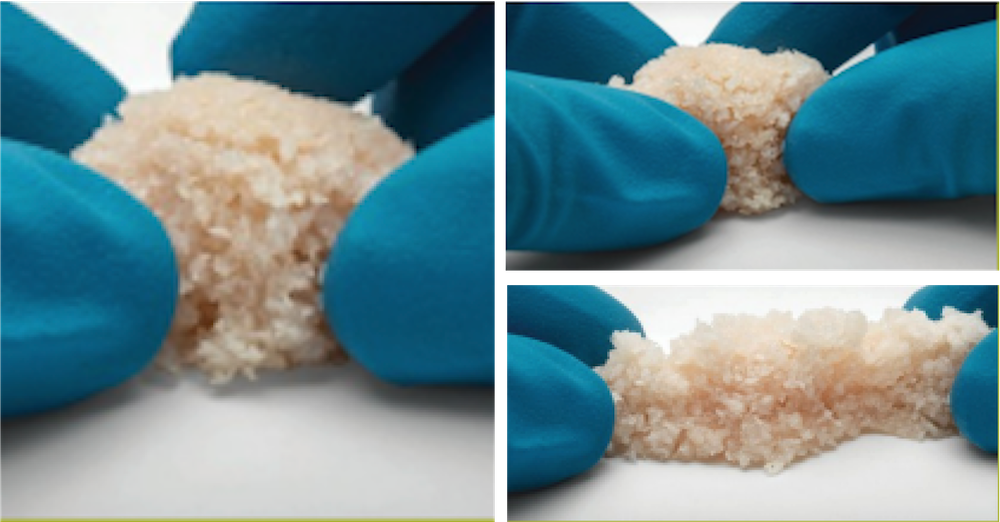
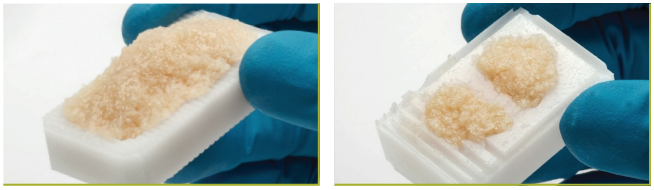
AlloFuse® Select CM provides extended working time up to 6 hours (4 hours in the state of New York), which allows the clinical staff to attend to the surgical procedure and not the product.
REFERENCES
1. Baboolal TG, Boxall SA, El-Sherbiny YM, et al. Multipotential stromal cell abundance in cellular bone allograft: comparison with fresh age-matched iliac crest bone and bone marrow aspirate. Regen Med 2014;9(5):593-607.
2. White Paper, Part #9501251A
3. Neman, et al. Lineage mapping and characterization of the native progenitor population in cellular bone allograft. The Spine Journal E-Pub 2013.
4. Williams GM, Moseley TA. Osteoinductivity of Allograft Cellular Bone Matrix. Spine J 2013;13:S98-S99.
5. Data on file
All necessary regulatory documentation, instructions for use, published studies, patient education tools, etc. (as applicable) can be provided upon request by contacting cs@spartanmedical.com or (888) 240-8091. We are available at any time to meet with providers and staff to answer any questions about Spartan Medical’s entire portfolio of advanced medical solutions.
Looking for more Advanced Biologics and Dermis solutions?
Click on the below links to see our entire portfolio