Griplasty™ System - Base of Thumb (BoT) With Needles
Next-generation CMC Suspensionplasty for the surgical management of thumb carpometacarpal (CMC) joint osteoarthritis
The Griplasty™ System Base of Thumb (BoT) with Needles is designed to provide a solution for patients with advanced-stage first carpometacarpal (CMC) joint osteoarthritis that relieves pain and restores thumb stability and strength.1,2 Symptomatic Thumb CMC OA occurs in up to 15% of the population aged 30 years or older, increasing to an estimated 33% of postmenopausal women.13 The Griplasty™ BoT procedure requires only a single incision allowing for either a volar or dorsal approach. The unique V-Sling of the Griplasty™ System BoT is designed to mitigate concerns of proximal migration of the first metacarpal by providing a broader surface area of support compared to traditional systems.2 The innovative deployment guidance* of the Griplasty™ System is designed to enable a precise, accurate and reproducible technique.2
- Intuitive, easy to use, tensioning construct.2
- Single incision site supporting a dorsal or volar surgical approach.5
- 'V-Sling' implant design with increased surface area of support designed to create a stronger construct.2
- Anchors deployed on the far cortex, above the area of degenerative bone which is designed to increase the construct's pull out strength and reduce risk of failure.2,3,4
- Compatible with trapeziectomy and tendon transfer procedures if clinically required.2,5
*Patent Pending
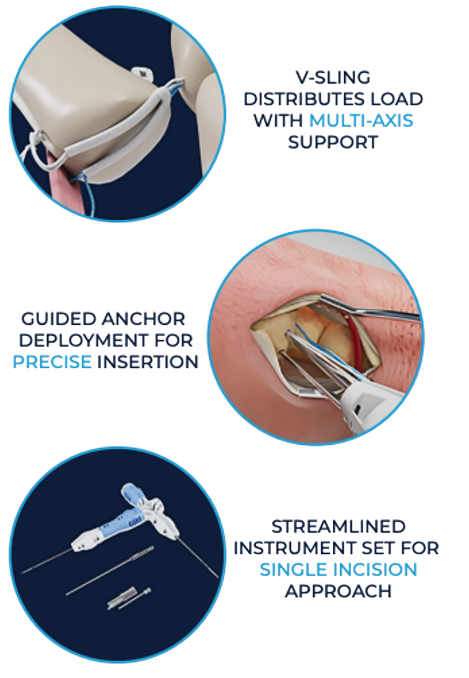
The streamlined instrument set enables a dorsal or volar single-incision approach, designed to support precise minimally invasive surgery.
Versatility - Ensuring a treatment option for a wide range of patients
The Griplasty™ System Base of Thumb with Needles is a part of the Extremity All Suture System. Indications for the Extremity All Suture System include the following: The Extremity All Suture System is intended to be used for suture or tissue fixation in the foot/ankle, knee, hand/ wrist, elbow, and shoulder. Specific indications are listed below:
- Elbow: Biceps tendon reattachment, ulnar or radial collateral ligament reconstruction.
- Shoulder: Rotator cuff repair, Bankart repair, SLAP lesion repair, Biceps tenodesis, Acromio-Clavicular Separation Repair, Deltoid Repair, Capsular Shift or Capsulolabral Reconstruction.
- Hand/Wrist: Scapholunate Ligament Reconstruction, Repair/reconstruction of collateral ligaments, Repair of flexor and extensor tendons at the PIP, DIP and MCP joint for all digits, digital tendon transfers, Carpal ligament reconstruction and carpometacarpal joint arthroplasty (Basal thumb joint arthroplasty).
- Foot/Ankle: Lateral stabilization, medial stabilization, achilles tendon repair, metatarsal ligament repair, hallux valgus reconstruction, digital tendon transfers, mid-foot reconstruction.
- Knee: Medial collateral ligament repair, lateral collateral ligament repair, patellar tendon repair, posterior oblique ligament repair, iliotibial band tenodesis.
System Components
The innovative* deployment guidance mechanism is designed to enable a precise, accurate and reproducible technique.2
- T-Handle preloaded with Anchors, Suture, V-Sling, and Needles (2)
- 1.2mm x 80mm Single-Ended Trajectory K-wires (2)
- K-wire Guide Sleeve and Parallel Wire Guide
- 1.6 mm x 90mm Single-Ended Guide K-wires (2)
- 1 x 2.4mm Cannulated Drill Bit
*Patent pending
.png)

Benefits of sterile single-use instruments
- Strong low profile suture anchors
- Minimizing soft tissue irritation.6
- Providing reliable fixation
- High pullout resistance with far cortex engagement.3
- Pre-loaded for quick insertion
- Streamlined Delivery
- Precise and pre-determined drill path.2
- Guided and accurate anchor placement.2,5
- Reproducible and consistent anchor deployment.2
- Anatomically contoured leading tip designed to improve intra operative positioning.2
- Sophisticated all-in-one system
- Pre-loaded and always one step ahead.
- Ergonomically designed T-Handle specifically tailored for the upper limb surgeon.2
- Streamlined and efficient workflow.7
- Clearly labeled for intuitive use
- Reliable product performance with new instruments for every case.8
- Environmentally conscious and cost efficient single use packaging to optimize the perioperative process.9,10,11,12
REFERENCES
1. Larsen CG et al. Knotless suture anchor suspensionplasty for basal joint arthritis: a prospective case series with short-term outcomes. Bull Hosp Jt Dis.2022;80(2):129-3
2. Data on file.
3. Hozack et al. Optimal Position of the Bone Anchor for the Internal Brace Suspensionplasty Technique for Thumb Basal Joint Arthroplasty. J Hand Surg Am. 2024 Apr;49(4):380.e6.doi:10.1016/j.jhsa.2022.08.001. Epub 2022 Sep 10. PMID: 36100487.
4. Oh J H et al. Pullout Strength of All-Suture Anchors: Effect of the Insertion and Traction Angle - A Biomechanical Study. Arthroscopy. 2018 Oct;34(10):2784-2795 doi:10.1016/j.arthro.2018.04.028. Epub 2018 Sep 1. PMID: 30181056.
5. Field Orthopaedics. (2024). GriplastyTM Surgical Technique. Brisbane, Australia: Field Orthopaedics.
6. Ergun S et al. The Clinical and Biomechanical Performance of All-Suture Anchors: A Systematic Review. Arthroscopic Sports Medicicine and Rehabilitation. 2020 May;28;2(3);e263-e275. doi: 10.1016/j.asmr.2020.02.007. PMID: 32548592; PMCID:PMC7283965.
7. Wong J et al. Delays in the Operating Room: Signs of an Imperfect System. Canadian Journal of Surgery. 2010 Jun;53:189-194.
8. Hale L. Developing Devices to Meet Today's Orthopaedic Trends - An Orthopaedic Innovators Q&A (ed.) Sean Fenske. Orthopaedic Design & Technology, Oct 28, 2022.
9. Leiden A et al. Life Cycle Assessment of a Disposable and a Reusable Surgery Instrument Set for Spinal Fusion Surgeries. Resources, Conservation & Recycling. 2020;156
10. Yasser, A. Value Based Healthcare: Maximizing Efficacy and Managing Risk with Spinal Implant Technology. Interdisciplinary Neurosurgery. 2020;22.
11. Agarwal A et al. A Paradigm Shift Toward Terminally Sterilized Devices. Clinical Spine Surgery. 2018;31:308-311.
12. Goldberg, TD et al. Logistical and Economic Advantages of Sterile-Packed, Single-Use Instruments for Total Knee Arthroplasty. Journal of Arthroplasty. 2019;34:1876-1883
13. Higgenbotham C, Boyd A, Busch MJ, Trumble TE. Optimal management of thumb basal joint arthritis: challenges and solutions. J Hand Surg Am. 2018 Mar;43(3):e101–e109. doi:10.1016/j.jhsa.2017.11.018.
All necessary regulatory documentation, instructions for use, published studies, patient education tools, etc. (as applicable) can be provided upon request by contacting cs@spartanmedical.com or (888) 240-8091. We are available at any time to meet with providers and staff to answer any questions about Spartan Medical’s entire portfolio of advanced medical solutions.
Looking for more Orthopedic solutions?
Click on the below links to see our entire portfolio



