Suprello™ Surgical Wound Matrix
Suprello™ is a peptide-based surgical wound matrix specifically formulated for use in high-risk cases, such as in patients with major comorbidities and complications (MCC) undergoing surgical procedures. Suprello™ is designed to help prevent wound dehiscence and associated surgical site complications.
Suprello™ peptides form a porous surgical wound matrix that enables cell migration, attachment, and proliferation while simultaneously offering broad-spectrum antibacterial protection effective against multidrug-resistant organisms (MDROs)1,2. The surgical wound matrix presents an optimal resorption profile to support tissue apposition at the re-approximated incision margins and to help promote sustained wound closure3,4.
.png)
How Suprello™ Works
Rapid Tissue Resorption for Sustained Wound Closure
Suprello™ surgical wound matrix rapidly resorbs and integrates into the host tissue to encourage healing by primary intention3. Suprello™ facilitates healing at the reapproximated incision margins by supporting complete tissue apposition and robust re-epithelialization for effective and sustained wound closure (see Figure 1).

Intimate Contact and Tissue Compatibility
Suprello™ flowable formulation offers optimal contact with the incision margins, creating an environment that supports healing through primary intention. The porous surgical wound matrix allows diffusion of oxygen and nutrients5, enabling cell infiltration at the surgical site to support wound healing6.
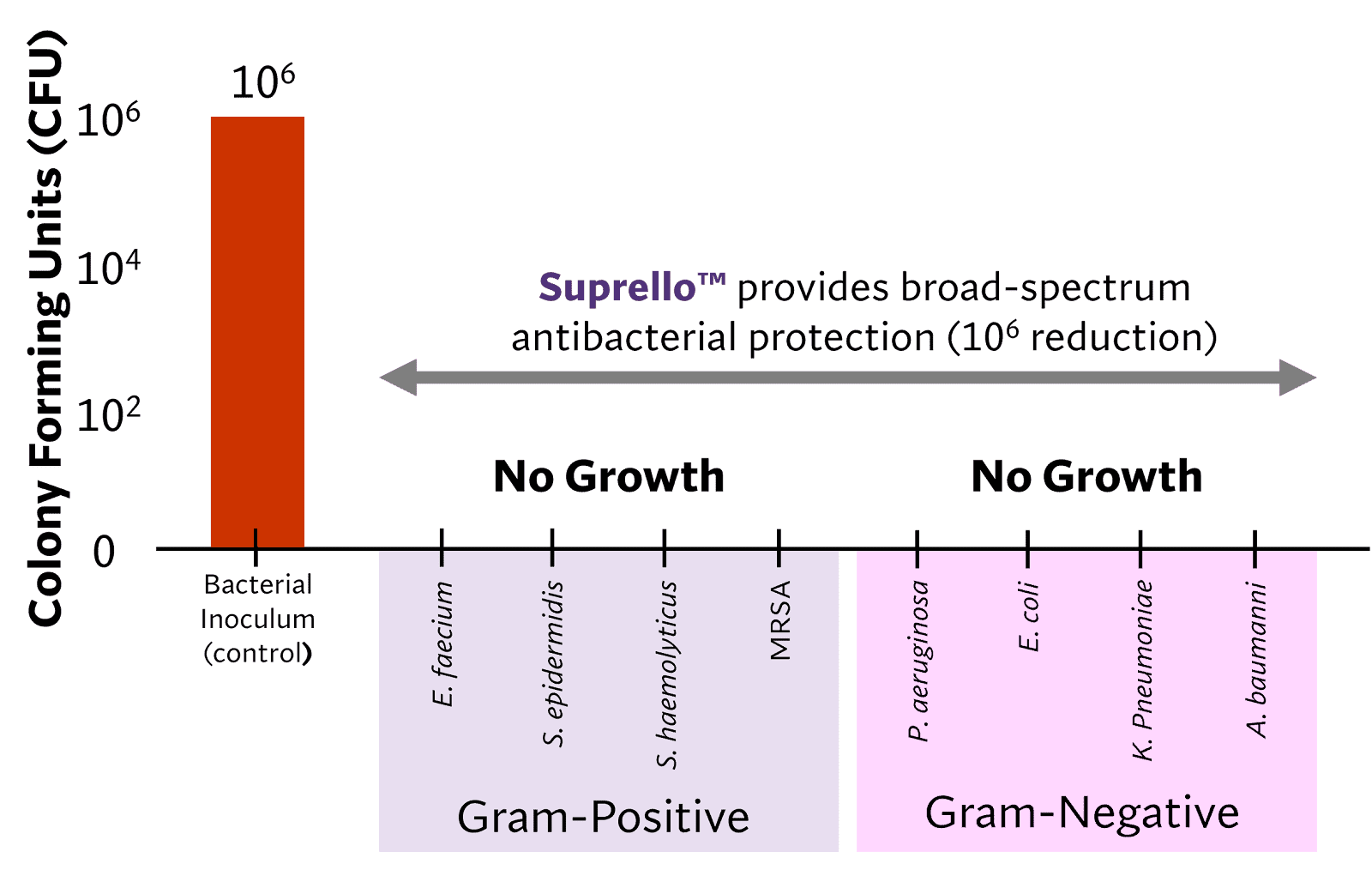
Strong Antibacterial Protection
Suprello™ provides inherent broad-spectrum antibacterial protection effective against multidrug-resistant organisms (MDROs)1, minimizing the risk of infection in high-risk surgical cases (see Figure 2). Suprello™ contains cationic peptides that provide strong antibacterial protection by interacting with and destabilizing bacterial membranes1,2. This innovative, physical mechanism of antibacterial protection overcomes local and systemic tissue toxicity while also minimizing the risk of antibacterial resistance, often associated with the use of antibiotics and antimicrobial agents.
Product Benefits
- Rapid tissue integration, supporting complete tissue apposition and sustained wound closure3.
- Strong broad-spectrum antibacterial protection against MDROs, minimizing the risk of developing infection in high-risk surgical cases1,2.
- Shelf-stable at room temperature for three years.
- Ready-to-use, eliminating the need for mixing, hydration, or reconstitution.
Suprello™ is FDA 510(k) cleared, and available by prescription only under the supervision of a healthcare professional for the local management of partial and full-thickness surgical wounds.
REFERENCES
1. Data on File, RR-0004
2. Data on File, RR-0003
3. Data on File (GM-2101)
4. FDA 510k submission package
5. Data on File, RR-0005
6. Data on File, RR-0001
All necessary regulatory documentation, instructions for use, published studies, patient education tools, etc. (as applicable) can be provided upon request by contacting cs@spartanmedical.com or (888) 240-8091. We are available at any time to meet with providers and staff to answer any questions about Spartan Medical’s entire portfolio of advanced medical solutions.
Looking for more Advanced Biologics and Dermis solutions?
Click on the below links to see our entire portfolio


