PureSkin™ Split-Thickness Dermis
Critical treatment solutions for burn management
PureSkin™ human skin allografts are temporary dressings used with critical burn patients to aid in infection control, fluid maintenance, and pain control, as well as to conserve the use of precious autograft skin. PureSkin™ is a partial thickness skin allograft, containing the epidermis and part of the dermis, including biologic factors native to human skin. The use of skin allografts in the management of burn wounds allow early excision and wound covering which may aid in the overall clinical management of the patient. Split-thickness skin allografts provide a crucial bridge to reconstruction, particularly for large total body surface area (TBSA) burns, which may be closed in a delayed manner.
- Native dermal growth factors remain present to aid in the healing process1
- Processed to maximize allograft area, minimizing “patchworking” of smaller pieces in clinical settings
- Choice of meshed allografts to save valuable OR time, or non-meshed allografts to allow custom adaptability (e.g., “pie crusting” or larger meshing patterns) at site
- Cryopreserved using a controlled rate freezing process and cryoprotectants to optimize shelf-life
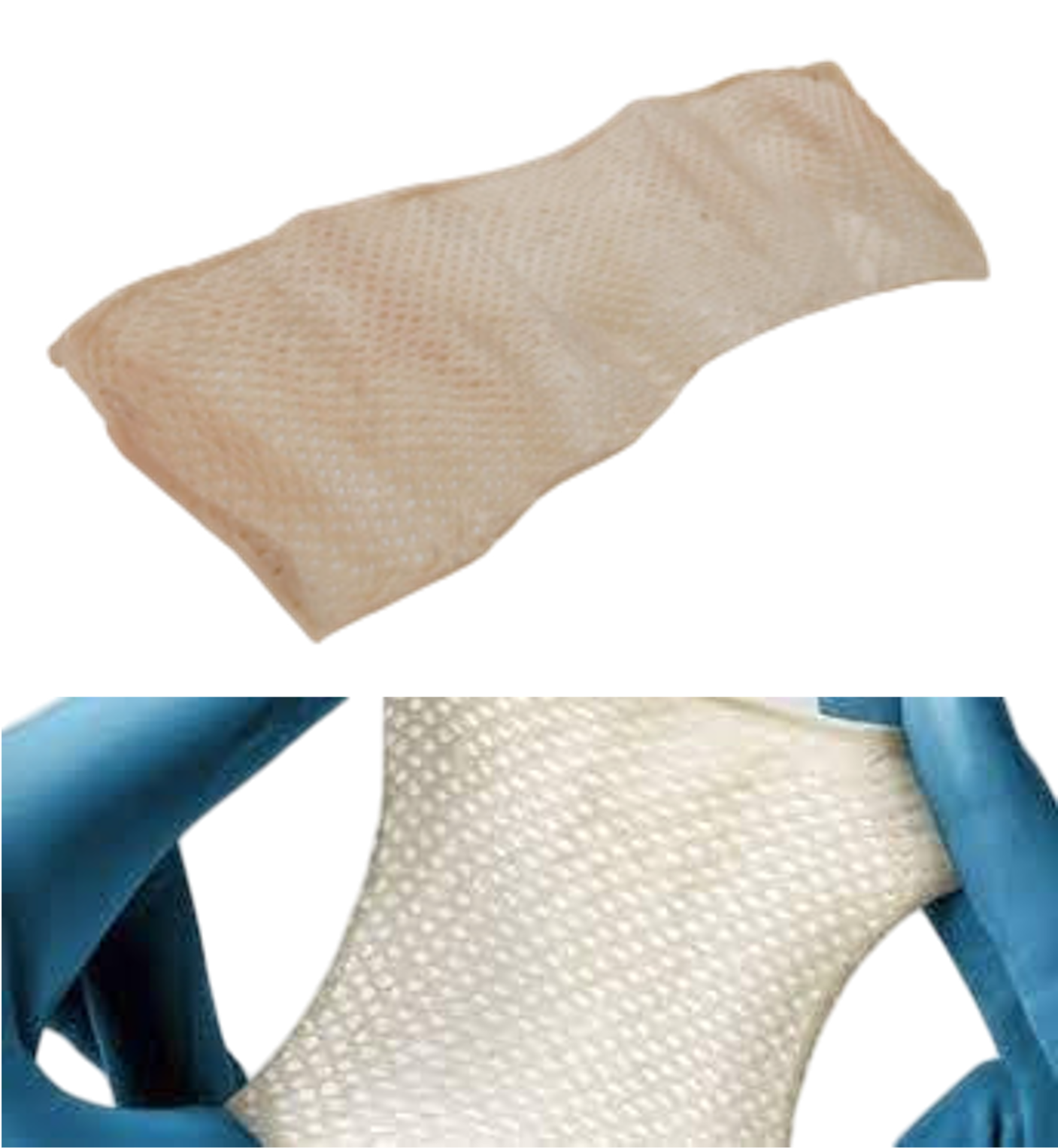
PureSkin™ XL Split-Thickness Dermis
- PureSkin™ XL allografts available with sizes up to 1800cm2 and greater
- Processed to maximize allograft area, minimizing “patchworking” of smaller pieces in clinical settings saving valuable OR time and reducing patient time under anesthesia in large TBSA burns, potentially reducing patient trauma with decreased suturing or stapling
- Meshing of 2:1 allows exudate management flexibility for diverse anatomical coverage and maximum expansion
- Utilizing S3 Skin Splitting Technology, allografts are split to exact thickness standards, ensuring consistency along the graft with no adipose tissue remnants to allow for uniform preparation of underlying burn bed
REFERENCES
1. Data on File at Allosource
All necessary regulatory documentation, instructions for use, published studies, patient education tools, etc. (as applicable) can be provided upon request by contacting cs@spartanmedical.com or (888) 240-8091. We are available at any time to meet with providers and staff to answer any questions about Spartan Medical’s entire portfolio of advanced medical solutions.
Looking for more Advanced Wound Care solutions?
Click on the below links to see our entire portfolio
- NATROX® O₂ Continuous Topical Oxygen Therapy
- APIS® Synthetic Wound Matrix
- Microlyte Bioresorbable Matrix
- AlloSkin™ Meshed Human Dermal Graft
- Amnion: Membrane
- XSONX® Wound Hygiene System
- G4Derm™ Plus Flowable Biomimetic Matrix

.png)

