Elute, Inc. and Spartan Medical Announce Strategic Partnership
Elute, Inc., an emerging leader in drug-eluting device technology for controlled and extended drug release, today announced a strategic partnership with Spartan Medical Inc., a Service-Disabled Veteran-Owned and operated medical solutions company serving the Department of Veterans Affairs (VA), Department of Defense (DoD), civilian hospitals, and Ambulatory Surgery Centers (ASCs) nationwide. This partnership will expand access of Elute's BonVie+™ bone graft substitute to physicians and patients at VA and DoD medical facilities worldwide.

SALT LAKE CITY and ROCKVILLE, Md., Sept. 9, 2025 /PRNewswire/ -- Elute, Inc., an emerging leader in drug-eluting device technology for controlled and extended drug release, today announced a strategic partnership with Spartan Medical Inc., a Service-Disabled Veteran-Owned and operated medical solutions company serving the Department of Veterans Affairs (VA), Department of Defense (DoD), civilian hospitals, and Ambulatory Surgery Centers (ASCs) nationwide. This partnership will expand access of Elute's BonVie+™ bone graft substitute to physicians and patients at VA and DoD medical facilities worldwide.
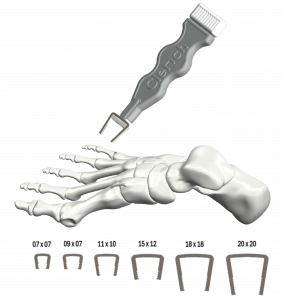
Bone graft substitutes are used to fill bone voids caused by trauma or infection. Traditional options such as bone cement and calcium sulfate-based granules can have significant drawbacks. Bone cement is non-biodegradable, does not support new bone growth, and often requires a second surgery for removal. Calcium sulfate substitutes resorb naturally but can cause fluid buildup (seromas) and wound drainage.
Elute'sBonVie+™ was developed to overcome these challenges. The implant gradually resorbs while being replaced by the patient's own healthy bone, creating a more natural and predictable path to healing. "This new era in resorbable bone graft fillers represents over a decade of innovation, equipping clinicians with unmatched consistency in bone restoration and reliable outcomes for patients," said Ashok Khandkar, Ph.D., CEO of Elute.
Spartan Medical Inc. is also leading a national campaign to raise awareness on the consequences of surgical site infections. BonVie+™ complements Spartan's efforts by providing a better solution to address sub optimal bone healing caused by infections. While orthopedic surgeries are highly effective at restoring joint function and mobility, their infection rates can range from 1%–5%. Complications often require prolonged hospitalization, multiple surgeries, and place a significant burden on healthcare systems. As part of its mission, Spartan Medical delivers best-in-class products such as BonVie+™ to help improve patient outcomes and reduce complications.
"In a marketplace filled with fusion based biologics, the differentiation must be unique and solve real problems our surgeons are dealing with. BonVie+™ creates an advantageous environment for complex orthopedic procedures…optimal resorption rate on the fusion side without seromas and wound drainage, which can complicate recovery, increase infection risk, and can interfere with implant or bone healing," said Vince Proffitt, Founder & President of Spartan Medical. "Through our firm, fixed, fair pricing on two nationwide federal contracts, and a renewed prioritization of home-grown manufacturing, Spartan is thrilled to add another advanced medical technology that is proudly made right here in America." Through innovation and partnership with Elute Inc, Spartan fulfills its mission of providing the world's finest medical products to our nation—in particular our soldiers, veterans, and their families.
About BonVie+™
BonVie+™ is a calcium-salt resorbable bone void filler implant used to fill bone defects, and with time, is replaced by new bone into the space previously occupied by the implant.
About Elute, Inc.
Elute, Inc. is an emerging leader in the development of its novel drug eluting device platform for controlled and extended release of drugs. Elute's lead product, EP Granules with Tobramycin, is similar to BonVie+™ but is pre-loaded with tobramycin, which is released over 8-weeks in a locally targeted region while allowing growth of new bone. This is an investigational device limited to investigational use in the United States. BonVie+™ is FDA cleared and commercially available now. www.elutinc.com.
About Spartan Medical Inc.:
Spartan Medical is a veteran-owned-and-operated government contractor for the U.S. Department of Veterans Affairs, the U.S. Department of Defense, and other local, state, and federal agencies. All senior executives have held leadership positions in both the public and private sector and employ the core strengths of solving complex problems through creative thinking, innovative solutions, and highly skilled flexible teams. Spartan Medical maintains its vanguard position by providing medical facilities with A-to-Z solutions that improve patient care and outcomes, especially for America's military and veteran communities. For ordering, specification, or further information, please contact our Customer Service team at cs@spartanmedical.com or call 888-240-8091.
Contact Information
Ashok Khandkar, Ph.D., President and CEO Elute, Inc.
(801) 410-4330
In the spotlight

Spartan Medical Deploys A-Z Wound Care Portfolio to Address Growing Number of Chronic Wounds in the Veteran Community